Buy The Probiotics Revolution - Microsoft Store
Microsoft Store
Get the The Probiotics Revolution at Microsoft Store and compare products with the latest customer reviews and ratings. Download or ship for free. Free returns.

Probiotics for prevention and treatment of respiratory... : Medicine
LWW
idence of or modify RTIs. The authors systematically reviewed data from randomized controlled trials (RCTs) to investigate the effect of probiotic consumption on RTIs in children.Methods:MEDLINE/PubMed, Embase, Cochrane Library, and Web of Science were systematically searched for RCTs regarding the effect of probiotics on RTIs in children. The outcomes included number of children experienced with at least 1 RTI episode, duration of illness episodes, days of illness per subject, and school/day care absenteeism due to infection. A random-effects model was used to calculate pooled relative risks, or mean difference (MD) with the corresponding 95% confidence interval (CI).Results:A total of 23 trials involving 6269 children were eligible for inclusion in the systematic review. None of the trials showed a high risk of bias. The quality of the evidence of outcomes was moderate. The age range of subjects was from newborn to 18 years. The results of meta-analysis showed that probiotic consumption significantly decreased the number of subjects having at least 1 RTI episode (17 RCTs, 4513 children, relative risk 0.89, 95% CI 0.82–0.96, P = 0.004). Children supplemented with probiotics had fewer numbers of days of RTIs per person compared with children who had taken a placebo (6 RCTs, 2067 children, MD −0.16, 95% CI −0.29 to 0.02, P = 0.03), and had fewer numbers of days absent from day care/school (8 RCTs, 1499 children, MD −0.94, 95% CI −1.72 to −0.15, P = 0.02). However, there was no statistically significant difference of illness episode duration between probiotic intervention group and placebo group (9 RCTs, 2817 children, MD −0.60, 95% CI −1.49 to 0.30, P = 0.19).Conclusion:Based on the available data and taking into account the safety profile of RCTs, probiotic consumption appears to be a feasible way to decrease the incidence of RTIs in children. Background: Respiratory tract infections (RTIs) represent one of the main health problems in children. Probiotics are viable bacteria that colonize the intestine and affect the host intestinal microbial balance. Accumulating evidence suggests that probiotic consumption may decrease the incidence of or modify RTIs. The authors systematically reviewed data from randomized controlled trials (RCTs) to investigate the effect of probiotic consumption on RTIs in children.Methods: MEDLINE/PubMed, Embase, Cochrane Library, and Web of Science were systematically searched for RCTs regarding the effect of probiotics on RTIs in children. The outcomes included number of children experienced with at least 1 RTI episode, duration of illness episodes, days of illness per subject, and school/day care absenteeism due to infection. A random-effects model was used to calculate pooled relative risks, or mean difference (MD) with the corresponding 95% confidence interval (CI).Results: A total of 23 trials involving 6269 children were eligible for inclusion in the systematic review. None of the trials showed a high risk of bias. The quality of the evidence of outcomes was moderate. The age range of subjects was from newborn to 18 years. The results of meta-analysis showed that probiotic consumption significantly decreased the number of subjects having at least 1 RTI episode (17 RCTs, 4513 children, relative risk 0.89, 95% CI 0.82–0.96, P = 0.004). Children supplemented with probiotics had fewer numbers of days of RTIs per person compared with children who had taken a placebo (6 RCTs, 2067 children, MD −0.16, 95% CI −0.29 to 0.02, P = 0.03), and had fewer numbers of days absent from day care/school (8 RCTs, 1499 children, MD −0.94, 95% CI −1.72 to −0.15, P = 0.02). However, there was no statistically significant difference of illness episode duration between probiotic intervention group and placebo group (9 RCTs, 2817 children, MD −0.60, 95% CI −1.49 to 0.30, P = 0.19).Conclusion: Based on the available data and taking into account the safety profile of RCTs, probiotic consumption appears to be a feasible way to decrease the incidence of RTIs in children....

probiotics Archives | The Nature Doctors
The Nature Doctors
All Nature Doctors Naturopathic Family Medicine Inc news posts tagged under probiotics
Suggested protocol to determine amount of mite immigration
by Randy @ Scientific Beekeeping
Sun Feb 11 13:11:02 PST 2018
Suggested protocol to determine amount of mite immigration Randy Oliver 8 February 2018 The methodology for quantifying the number of mites invading a hive per time period is relatively straightforward: At least six weeks in advance (I suggest mid May), choose one or more strong, healthy hives to monitor. In order to avoid inadvertently selecting […]
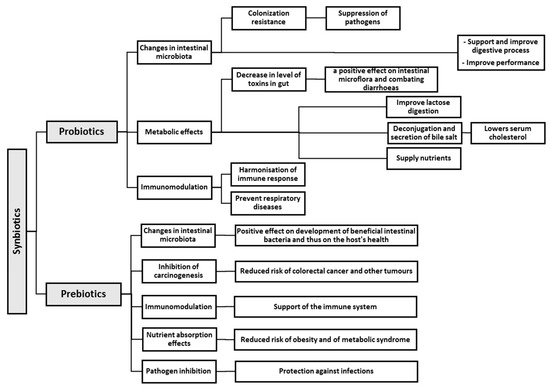
Effects of Probiotics, Prebiotics, and Synbiotics on Human Health
MDPI
The human gastrointestinal tract is colonised by a complex ecosystem of microorganisms. Intestinal bacteria are not only commensal, but they also undergo a synbiotic co-evolution along with their host. Beneficial intestinal bacteria have numerous and important functions, e.g., they produce various nutrients for their host, prevent infections caused by intestinal pathogens, and modulate a normal immunological response. Therefore, modification of the intestinal microbiota in order to achieve, restore, and maintain favourable balance in the ecosystem, and the activity of microorganisms present in the gastrointestinal tract is necessary for the improved health condition of the host. The introduction of probiotics, prebiotics, or synbiotics into human diet is favourable for the intestinal microbiota. They may be consumed in the form of raw vegetables and fruit, fermented pickles, or dairy products. Another source may be pharmaceutical formulas and functional food. This paper provides a review of available information and summarises the current knowledge on the effects of probiotics, prebiotics, and synbiotics on human health. The mechanism of beneficial action of those substances is discussed, and verified study results proving their efficacy in human nutrition are presented.
Assessment of Maternal Nifedipine as a Tocolytic Agent on the Doppler Indices of Uterine and Fetal Umbilical and Middle Cerebral Arteries
by opeditor @ Openventio Publishers
Wed Jan 10 23:06:12 PST 2018
Recognizing and Avoiding Common Household Toxins
by Zohair @ Dr. Group's Healthy Living Articles
Thu Dec 14 08:00:51 PST 2017
Many people don’t realize the number of toxins they are surrounded by in their home. According to the Centers for Disease Control and Prevention, the air in homes and other buildings is usually more polluted than the air outdoors, even in major cities. With many new chemicals introduced to the world’s markets every year, household toxins are more prevalent now than ever before. These toxins are found in many household items, including mattresses, floors, furniture, and cosmetics. Ridding your house of these harmful substances can make your home healthier, happier, […]
The post Recognizing and Avoiding Common Household Toxins appeared first on Dr. Group's Healthy Living Articles.
Dosimetric Characterization of an Abscopal Response in a Patient With Oligometastatic Melanoma Undergoing Concurrent Treatment With Pembrolizumab and Stereotactic Body Radiotherapy (SBRT)
by opeditor @ Openventio Publishers
Wed Jan 31 06:23:45 PST 2018

What Are Prebiotics?
Dr. Group's Healthy Living Articles
Prebiotics are a type of fiber that is food for probiotics, the organisms that live in the gut. Here we’ll explore the prebiotic/probiotic relationship.
What the Bristol Stool Scale Tells You About Your Poop
by Zohair @ Dr. Group's Healthy Living Articles
Thu Jan 11 08:00:29 PST 2018
Your poop is an important indicator of how your body is running and your current health status. While using the appearance of your poop to gauge your wellness may seem strange to some, it is a medically proven way to detect an imbalance in your gut and disruptions to your digestive health. If you want to measure your health by your poop, then the Bristol Stool Scale is one of the best tools to use. What Is the Bristol Stool Scale? The Bristol Stool Scale, sometimes called the Bristol Stool […]
The post What the Bristol Stool Scale Tells You About Your Poop appeared first on Dr. Group's Healthy Living Articles.

Genome Sequences of Potential Probiotic Lactobacillus rhamnosus Isolates from Human Infants
PubMed Central (PMC)
Probiotics provide health benefits to their hosts, including modulation of host immune response, inhibition of colonization by pathogens, modulation of the gut microbiota, and epithelial barrier enhancement. Here, we present the draft genome sequences ...
Refusal of Venous Thromboembolism Prophylaxis and Incidence of Thrombosis in Patients with Cystic Fibrosis
by opeditor @ Openventio Publishers
Thu Dec 28 06:18:28 PST 2017

Probiotics in Asthma and Allergy Prevention
PubMed Central (PMC)
Interest in probiotic research and its potential benefits in infant foods are relatively recent but significantly increasing. The evolution of the knowledge in the last 20 years demonstrated that alterations in the microbiome may be a consequence ...

7 Skin-Fixing Probiotic & Protein Supplements For Women - Charlottes Book
Charlottes Book
The benefits of supplements—protein, probiotic, fiber—aren't reserved for body builders. Read on for a list of supplements that will glow you away.

Probiotics and Prebiotics (PDF Download Available)
ResearchGate
Full-text (PDF) | Cereal Chem. 80(2):113–117 Probiotics, bacteria from the genera Bifidobacterium and Lacto-bacillus, and yeast, Saccharomyces, as well as prebiotics belonging to the group of dietary fiber (inulin with low degree of polymerization, fructose-derived oligosaccharides, and resistant...

A randomised controlled demonstration trial of multifaceted nutritional intervention and or probiotics: the healthy mums and babies (HUMBA) trial
BMC Pregnancy and Childbirth
Maternal obesity is associated with adverse pregnancy outcomes and has lifelong negative implications for offspring health. The Institute of Medicine recommends limited gestational weight gain (GWG) in obese women for optimal maternal and infant outcomes. However, there is a gap regarding an effective and sustainable intervention strategy to achieve this goal. The aim of the healthy mums and babies (HUMBA) demonstration trial is to assess whether a multifaceted nutritional intervention and/or an oral probiotic treatment in obese pregnant women can reduce excessive GWG and optimise pregnancy outcomes. The study is a two by two factorial randomised controlled demonstration trial conducted in Counties Manukau health region, New Zealand, a multi-ethnic region with a high prevalence of obesity. A total of 220 non-diabetic obese women with a singleton pregnancy will be recruited between 120 and 176 weeks. At recruitment, women are randomised to receive either a culturally tailored multifaceted dietary intervention or routine dietary advice, and either an oral probiotic or placebo capsule. Randomisation is undertaken via a web-based protocol, randomize.net, with a 1:1 ratio using stratification by body mass index (BMI) category (BMI of 30–34.9 or BMI ≥35 kg/m2). The dietary intervention includes 4 customised nutrition education visits by a trained community health worker combined with motivational text messaging. Probiotic capsules consist of Lactobacillus rhamnosus GG and Bifidobacterium lactis BB12 at a dose of 7 × 109 colony-forming units one per day until birth. Probiotic and placebo capsules are identically pre-packed and labelled by a third party, and are prescribed in a double blinded fashion. Research assessments are conducted at enrolment, 28 weeks, 36 weeks, at birth and at 5 months post-delivery. The primary outcomes for the study are proportion of women with excessive GWG and infant birthweight. The HUMBA demonstration trial will assess the efficacy of a culturally tailored multifaceted dietary intervention and probiotic treatment in limiting excessive GWG and optimising birthweight in a multiethnic sample of obese pregnant women. If successful, either one or both of the interventions may be incorporated into future studies powered to investigate important pregnancy outcomes. Australian New Zealand Clinical Trials Registry registration number: ACTRN12615000400561 , Universal Trial Number: U1111-1155-0409. Date registered: 29th April 2015.